Cancer From Fasting?
The Role of Fasting, Refeeding, and the Development of Tumors in the Intestine
Studies are the key to understanding
Bottom Line Upfront
The study found that while fasting can help improve health and tissue repair, refeeding after fasting might increase the risk of cancer, especially in the intestines. This happens because refeeding makes certain cells in the intestines grow rapidly, which can lead to tumors, particularly if there's a loss of the Apc gene, a key tumor-suppressor. The process is driven by a pathway called mTORC1, which boosts protein production during refeeding. This shows that while fasting has benefits, the refeeding phase needs careful management to avoid increasing cancer risk.
Introduction
For over a century, fasting has been praised for its ability to improve health, increase lifespan, and aid in tissue regeneration across various species, including humans. From short-term fasting to more prolonged periods, these dietary interventions have shown numerous benefits. However, recent studies have delved deeper into how fasting and the subsequent refeeding process can affect adult stem cells in the intestines and potentially lead to the development of tumors. In this article, we'll explore the findings from a recent study that sheds light on these important mechanisms.
Thanks to Amber O'Hearn for sharing another great article that highlights the critical relationship between fasting, refeeding, and tumor formation. This study helps us understand how different dietary strategies might influence the risk of developing cancer, particularly in the intestines.
Understanding the Basics: What Is Fasting and Refeeding?
Before diving into the study, it’s essential to understand what fasting and refeeding mean in this context. Fasting refers to the voluntary abstention from food for a specific period. During fasting, the body enters a state where it begins to break down its energy reserves, such as glycogen stored in the liver and muscles, and eventually fats and proteins. This process is known as catabolism.
Refeeding occurs when food is reintroduced after a period of fasting. This phase is crucial because it triggers various physiological responses in the body, particularly in the intestines, where stem cells become highly active. According to the documentation from the trial, the refeeding phase after fasting is a distinct state that significantly impacts the behavior of intestinal stem cells (ISCs) and the potential for tumor development.
The Experiment: How the Study Was Conducted
To investigate the effects of fasting and refeeding on intestinal stem cells and tumor formation, researchers conducted a series of experiments using mice. The mice were divided into several groups:
Ad libitum group: These mice had constant access to food, meaning they ate whenever they wanted.
Fasted group: These mice were deprived of food for 24 hours but had access to water.
Refed 1-day group: After 24 hours of fasting, these mice were given food again and observed for one day.
Refed 3-day group: Similar to the refed 1-day group, but these mice were observed for three days after refeeding.
The researchers were particularly interested in how these different dietary conditions would affect the proliferation of ISCs and the formation of tumors, especially in mice that had a genetic predisposition for cancer due to the loss of the Apc gene.
What Is the Apc Gene?
Let me explain in simpler terms: The Apc gene is like a brake in the body’s cellular machinery. It helps to prevent cells from growing and dividing uncontrollably. In healthy cells, the Apc gene ensures that the WNT signaling pathway—a critical pathway for controlling cell growth—is kept in check. However, when the Apc gene is lost or mutated, this control is lost, and cells can begin to grow out of control, leading to the formation of tumors, particularly in the intestines and colon.
Key Findings of the Study
The results of the study were striking and demonstrated a clear link between the refeeding process and an increased risk of tumor formation. Here are the key findings:
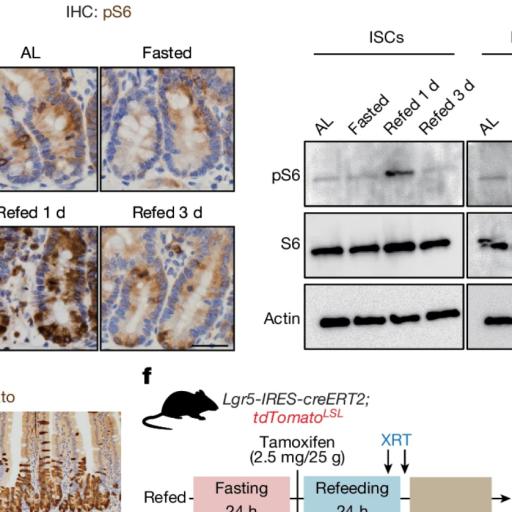
Increased ISC Proliferation During Refeeding: During the fasting period, the proliferation of ISCs (the cells that can turn into other cell types and are crucial for tissue repair) was reduced. However, when food was reintroduced (refeeding), these cells began to proliferate rapidly. This increase was much more significant in the refed groups compared to the fasted or ad libitum-fed groups.
Tumor Formation Linked to Refeeding: The study found that refeeding after fasting led to a higher incidence of tumors in the small intestine and colon, especially in mice that lacked the Apc gene. This suggests that while fasting might have protective effects by reducing cell proliferation, the refeeding phase can trigger an environment that promotes tumor growth.
The Role of the mTORC1 Pathway: One of the critical mechanisms identified in the study was the activation of the mTORC1 pathway during refeeding. This pathway is essential for regulating cell growth and protein synthesis. The researchers found that refeeding led to a robust induction of mTORC1 activity in ISCs, which in turn increased protein synthesis through a process called polyamine metabolism. Blocking this pathway reduced the regenerative and tumorigenic effects of refeeding, highlighting its central role in these processes.
I will break this down for you here: The mTORC1 pathway acts like a switch that turns on when the body starts eating again after fasting. This switch boosts the production of proteins that the cells need to grow and repair tissues. But when this pathway is too active, especially in cells that have lost the Apc gene, it can also lead to the uncontrolled growth of cells, resulting in tumors.
Implications for Fasting and Refeeding Strategies
These findings have significant implications for anyone considering fasting as part of a health regimen. While fasting has been shown to have numerous benefits, including enhanced tissue regeneration and potentially even reducing the risk of certain diseases, the refeeding phase must be approached with caution. These seem to be the key factors in creating the proper conditions for tumor development: the timing of refeeding, the activation of pathways like mTORC1, and the presence of genetic mutations like the loss of the Apc gene.
The study suggests that while fasting can slow down cell proliferation, refeeding—if not carefully managed—can lead to a burst of cellular activity that increases the risk of cancer, particularly in individuals with a predisposition to the disease. This highlights the need for further research and careful planning when incorporating fasting into dietary strategies aimed at promoting health and longevity.
The WNT Signaling Pathway: A Closer Look
As mentioned earlier, the WNT signaling pathway is critical in regulating cell growth and division. In healthy cells, this pathway is tightly controlled by the Apc gene, ensuring that cells only grow and divide when necessary. However, when the Apc gene is mutated or lost, the WNT signaling pathway can become overactive, leading to uncontrolled cell proliferation and tumor formation.
The study showed that during the refeeding phase, the WNT signaling pathway becomes particularly active, especially in ISCs. This increased activity, combined with the loss of the Apc gene, creates a perfect storm for tumor development. The findings underscore the importance of understanding how different signaling pathways interact during dietary interventions like fasting and refeeding.
The Role of Polyamines in Tumor Formation
Another critical aspect of the study was the role of polyamines in the process of tumor formation. Polyamines are organic compounds that play a crucial role in cell growth and function. During the refeeding phase, the production of polyamines increases significantly, driven by the activation of the mTORC1 pathway.
The study found that inhibiting the production of polyamines could reduce the tumorigenic effects of refeeding, suggesting that polyamines are a key factor in this process. This finding opens up new avenues for research into how targeting polyamine metabolism might help prevent or treat cancers associated with dietary interventions like fasting and refeeding.
Conclusion
In summary, the study provides valuable insights into the complex relationship between fasting, refeeding, and tumor development in the intestines. While fasting has been shown to have many health benefits, the refeeding phase presents a potential risk, particularly for individuals with a genetic predisposition to cancer. The study highlights the need for careful consideration and planning when incorporating fasting into a health regimen, especially when the goal is to promote tissue regeneration without increasing cancer risk.
This research also underscores the importance of further studies to explore how different dietary strategies can be optimized to maximize their benefits while minimizing their risks. By understanding the underlying mechanisms—such as the role of the mTORC1 pathway and polyamine metabolism—we can better design dietary interventions that promote health and longevity without inadvertently increasing the risk of diseases like cancer.
I used AI from AmazonAWS to re-write this article for better readability and comprehension. I hope it helps.
The study found that while fasting can help improve health and tissue repair, refeeding after fasting might increase the risk of cancer, especially in the intestines. This happens because refeeding makes certain cells in the intestines grow rapidly, which can lead to tumors, particularly if there's a loss of the Apc gene, a key tumor-suppressor. The process is driven by a pathway called mTORC1, which boosts protein production during refeeding. This shows that while fasting has benefits, the refeeding phase needs careful management to avoid increasing cancer risk.
Introduction
For over a century, fasting has been praised for its ability to improve health, increase lifespan, and aid in tissue regeneration across various species, including humans. From short-term fasting to more prolonged periods, these dietary interventions have shown numerous benefits. However, recent studies have delved deeper into how fasting and the subsequent refeeding process can affect adult stem cells in the intestines and potentially lead to the development of tumors. In this article, we'll explore the findings from a recent study that sheds light on these important mechanisms.
Thanks to Amber O'Hearn for sharing another great article that highlights the critical relationship between fasting, refeeding, and tumor formation. This study helps us understand how different dietary strategies might influence the risk of developing cancer, particularly in the intestines.
Understanding the Basics: What Is Fasting and Refeeding?
Before diving into the study, it’s essential to understand what fasting and refeeding mean in this context. Fasting refers to the voluntary abstention from food for a specific period. During fasting, the body enters a state where it begins to break down its energy reserves, such as glycogen stored in the liver and muscles, and eventually fats and proteins. This process is known as catabolism.
Refeeding occurs when food is reintroduced after a period of fasting. This phase is crucial because it triggers various physiological responses in the body, particularly in the intestines, where stem cells become highly active. According to the documentation from the trial, the refeeding phase after fasting is a distinct state that significantly impacts the behavior of intestinal stem cells (ISCs) and the potential for tumor development.
The Experiment: How the Study Was Conducted
To investigate the effects of fasting and refeeding on intestinal stem cells and tumor formation, researchers conducted a series of experiments using mice. The mice were divided into several groups:
Ad libitum group: These mice had constant access to food, meaning they ate whenever they wanted.
Fasted group: These mice were deprived of food for 24 hours but had access to water.
Refed 1-day group: After 24 hours of fasting, these mice were given food again and observed for one day.
Refed 3-day group: Similar to the refed 1-day group, but these mice were observed for three days after refeeding.
The researchers were particularly interested in how these different dietary conditions would affect the proliferation of ISCs and the formation of tumors, especially in mice that had a genetic predisposition for cancer due to the loss of the Apc gene.
What Is the Apc Gene?
Let me explain in simpler terms: The Apc gene is like a brake in the body’s cellular machinery. It helps to prevent cells from growing and dividing uncontrollably. In healthy cells, the Apc gene ensures that the WNT signaling pathway—a critical pathway for controlling cell growth—is kept in check. However, when the Apc gene is lost or mutated, this control is lost, and cells can begin to grow out of control, leading to the formation of tumors, particularly in the intestines and colon.
Key Findings of the Study
The results of the study were striking and demonstrated a clear link between the refeeding process and an increased risk of tumor formation. Here are the key findings:
Increased ISC Proliferation During Refeeding: During the fasting period, the proliferation of ISCs (the cells that can turn into other cell types and are crucial for tissue repair) was reduced. However, when food was reintroduced (refeeding), these cells began to proliferate rapidly. This increase was much more significant in the refed groups compared to the fasted or ad libitum-fed groups.
Tumor Formation Linked to Refeeding: The study found that refeeding after fasting led to a higher incidence of tumors in the small intestine and colon, especially in mice that lacked the Apc gene. This suggests that while fasting might have protective effects by reducing cell proliferation, the refeeding phase can trigger an environment that promotes tumor growth.
The Role of the mTORC1 Pathway: One of the critical mechanisms identified in the study was the activation of the mTORC1 pathway during refeeding. This pathway is essential for regulating cell growth and protein synthesis. The researchers found that refeeding led to a robust induction of mTORC1 activity in ISCs, which in turn increased protein synthesis through a process called polyamine metabolism. Blocking this pathway reduced the regenerative and tumorigenic effects of refeeding, highlighting its central role in these processes.
I will break this down for you here: The mTORC1 pathway acts like a switch that turns on when the body starts eating again after fasting. This switch boosts the production of proteins that the cells need to grow and repair tissues. But when this pathway is too active, especially in cells that have lost the Apc gene, it can also lead to the uncontrolled growth of cells, resulting in tumors.
Implications for Fasting and Refeeding Strategies
These findings have significant implications for anyone considering fasting as part of a health regimen. While fasting has been shown to have numerous benefits, including enhanced tissue regeneration and potentially even reducing the risk of certain diseases, the refeeding phase must be approached with caution. These seem to be the key factors in creating the proper conditions for tumor development: the timing of refeeding, the activation of pathways like mTORC1, and the presence of genetic mutations like the loss of the Apc gene.
The study suggests that while fasting can slow down cell proliferation, refeeding—if not carefully managed—can lead to a burst of cellular activity that increases the risk of cancer, particularly in individuals with a predisposition to the disease. This highlights the need for further research and careful planning when incorporating fasting into dietary strategies aimed at promoting health and longevity.
The WNT Signaling Pathway: A Closer Look
As mentioned earlier, the WNT signaling pathway is critical in regulating cell growth and division. In healthy cells, this pathway is tightly controlled by the Apc gene, ensuring that cells only grow and divide when necessary. However, when the Apc gene is mutated or lost, the WNT signaling pathway can become overactive, leading to uncontrolled cell proliferation and tumor formation.
The study showed that during the refeeding phase, the WNT signaling pathway becomes particularly active, especially in ISCs. This increased activity, combined with the loss of the Apc gene, creates a perfect storm for tumor development. The findings underscore the importance of understanding how different signaling pathways interact during dietary interventions like fasting and refeeding.
The Role of Polyamines in Tumor Formation
Another critical aspect of the study was the role of polyamines in the process of tumor formation. Polyamines are organic compounds that play a crucial role in cell growth and function. During the refeeding phase, the production of polyamines increases significantly, driven by the activation of the mTORC1 pathway.
The study found that inhibiting the production of polyamines could reduce the tumorigenic effects of refeeding, suggesting that polyamines are a key factor in this process. This finding opens up new avenues for research into how targeting polyamine metabolism might help prevent or treat cancers associated with dietary interventions like fasting and refeeding.
Conclusion
In summary, the study provides valuable insights into the complex relationship between fasting, refeeding, and tumor development in the intestines. While fasting has been shown to have many health benefits, the refeeding phase presents a potential risk, particularly for individuals with a genetic predisposition to cancer. The study highlights the need for careful consideration and planning when incorporating fasting into a health regimen, especially when the goal is to promote tissue regeneration without increasing cancer risk.
This research also underscores the importance of further studies to explore how different dietary strategies can be optimized to maximize their benefits while minimizing their risks. By understanding the underlying mechanisms—such as the role of the mTORC1 pathway and polyamine metabolism—we can better design dietary interventions that promote health and longevity without inadvertently increasing the risk of diseases like cancer.
I used AI from AmazonAWS to re-write this article for better readability and comprehension. I hope it helps.
Updated: August 13, 2025 10:19
References
"Short-term post-fast refeeding enhances intestinal stemness via polyamines". 21 August 2024. Imada, S. et al. https://www.nature.com/articles/s41586-024-07840-z (Paywall)