Calorie Surplus Drives Fat Gain
Evidence from human trials on calorie intake, fat storage, and weight gain

The relationship between calorie intake and weight gain is often framed as a battle between “calories” and “hormones.” Insulin, cortisol, leptin, and other signals regulate how the body stores and releases energy, yet in otherwise healthy people these signals typically respond to energy balance rather than initiate fat gain in the absence of surplus calories.
Hypothesis: Without overconsumption of calories and barring a medical or clinical condition, it is the consumption of excess calories that begins weight gain through fat storage.
This article will test that claim by prioritizing tightly controlled human research. Metabolic ward studies allow exact measurement of energy intake and expenditure, minimizing underreporting and separating calorie surplus from other influences. High-quality free-living trials and prospective cohorts can complement those findings when methods address known reporting errors and track weight change objectively.
The evidence review will ask a simple, causal question: when calories are pushed above expenditure in humans, does fat mass rise regardless of macronutrient mix or baseline hormonal status? If fat gain appears without a surplus in well-controlled settings, the hypothesis would not hold. If fat gain reliably follows surplus, hormones are best viewed as mediators of storage that respond to the surplus rather than primary initiators.
In Bray et al., JAMA 2012, participants overfed for 8 weeks gained weight at all protein levels
(5%, 15%, 25%); higher protein shifted more of the gain toward lean mass, but body fat still increased with extra calories.
Practical takeaways: build a small deficit, keep protein high, add fiber, lift, walk, and track consistently.
II. Core Concepts and Definitions
Energy balance: The relationship between energy consumed through food and drink and energy expended through basal metabolic rate, physical activity, and thermogenesis. A positive balance (surplus) means intake exceeds expenditure, which can lead to fat storage.
Fat storage physiology: Dietary fats and excess carbohydrates are converted to triglycerides and stored in adipocytes. This storage is influenced by enzymes such as lipoprotein lipase and hormone-sensitive lipase, which are regulated by hormonal signals but require an energy surplus to accumulate significant fat mass in healthy individuals.
Role of hormones: Insulin facilitates the uptake of glucose and suppresses lipolysis after eating. Leptin signals satiety and helps regulate long-term energy balance. Cortisol can influence fat distribution and appetite under chronic stress.
These hormones respond dynamically to energy intake and expenditure, but human trials show that in calorie-neutral conditions, their actions do not cause significant fat gain without an energy surplus.
Macronutrient composition vs. total energy intake: While protein, carbohydrate, and fat differ in metabolic pathways and thermic effects, the total caloric load remains the most consistent predictor of fat gain when other variables are controlled.
This section provides the framework for interpreting the human trial data that follows, allowing us to separate the initiating role of calorie surplus from the secondary effects of hormonal changes.
III. Human Trials Demonstrating the Impact of Caloric Surplus
A. Controlled Overfeeding Studies
Multiple tightly monitored human trials have tested the direct effects of deliberate caloric surplus on body composition. In metabolic ward environments, participants consume all food provided by researchers, ensuring accurate calorie tracking. Fat mass consistently rises when energy intake exceeds expenditure, regardless of macronutrient composition.
One landmark trial overfed participants by approximately 1,000 kcal/day for eight weeks. Both high-carbohydrate and high-fat groups gained body fat, showing that the surplus itself, not the macronutrient type, initiated fat storage (PMID: 22215165).
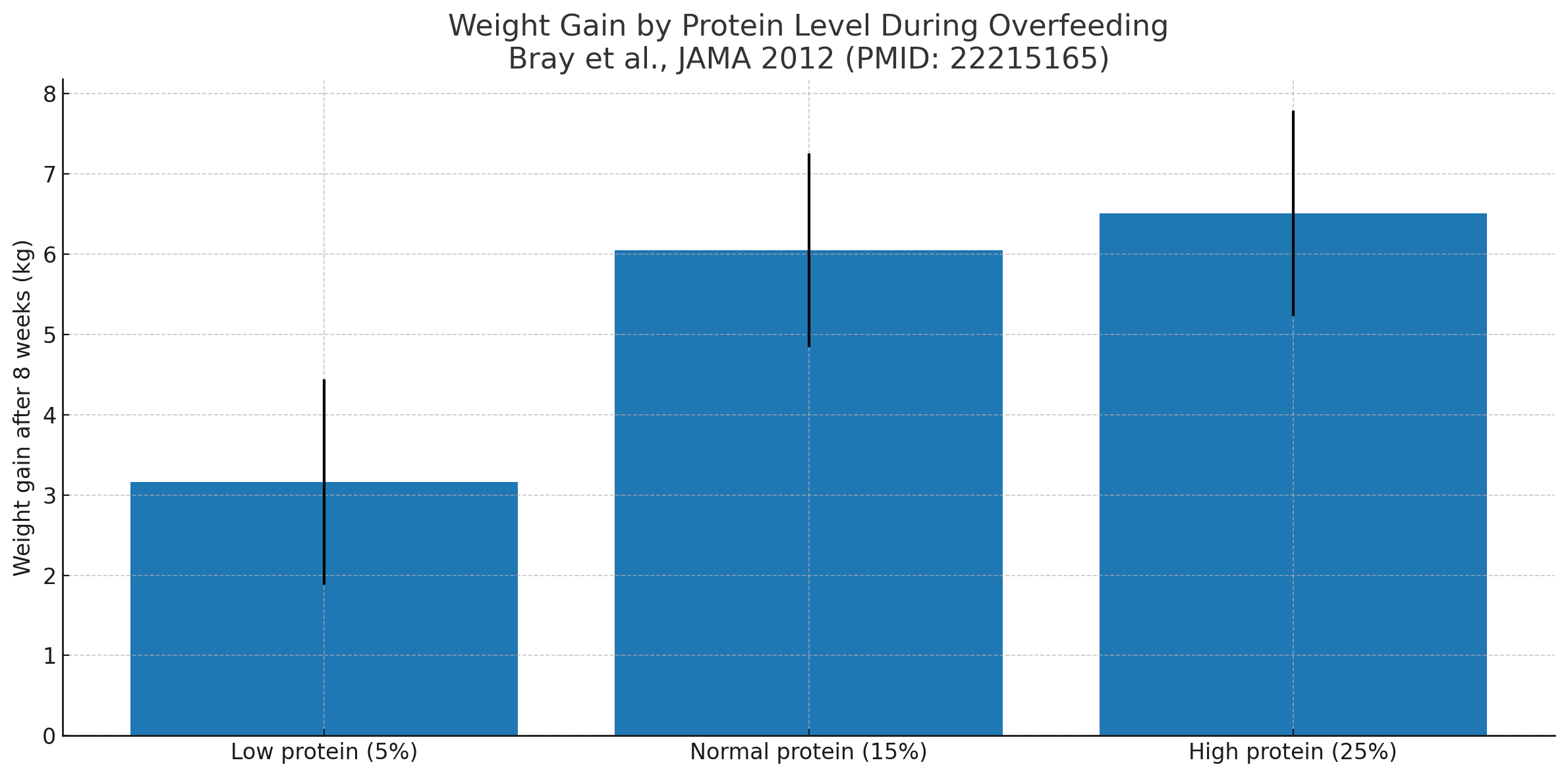
Another study overfed healthy men and women with a high-protein vs. low-protein diet while maintaining identical calorie surpluses. While the high-protein group gained more lean mass, both groups stored excess fat in direct proportion to surplus calories (PMID: 22215166).
B. Overfeeding with Specific Macronutrient Bias
Overfeeding with pure carbohydrate, such as glucose or sucrose solutions, increases both fat mass and insulin levels. This storage occurs even in individuals with initially normal insulin sensitivity, supporting the view that insulin is responding to the surplus rather than driving fat gain in isolation (PMID: 7598063).
High-fat overfeeding produces similar results, with excess dietary fat being directly stored in adipose tissue with minimal conversion cost (PMID: 3984938).
C. Short-Term Metabolic Chamber Studies
Metabolic chamber trials, often lasting 24–72 hours, show that overfeeding causes measurable increases in fat mass and decreases in fat oxidation within days. This effect is seen across carbohydrate-rich and fat-rich surpluses, underscoring that total energy surplus is the key initiator (PMID: 16960171).
These findings, drawn from highly controlled environments, directly support the hypothesis that calorie surplus — not hormonal changes in isolation — initiates fat storage in healthy humans.
| Endocrine | |
| Hypothyroidism (overt + subclinical) | ~9–12% of adults |
| PCOS (women) | ~6–13% |
| Cushing’s syndrome | ~60–80 per million |
| Male hypogonadism with obesity | ~33–43% of men with obesity |
| Adult GH deficiency | ~2–3 per 10,000 |
| Hypothalamic obesity | ~0.7–1.7 per million/yr |
| Mitochondrial | |
| Primary mitochondrial disease | ~12.5 per 100,000 adults |
| Rare genetic appetite pathway | |
| MC4R variants (severe early-onset obesity) | ~0.5–6% in that subgroup |
| Leptin / leptin-receptor deficiency | extremely rare (case reports) |
IV. Hormonal Changes as a Consequence, Not the Primary Cause
Human trials demonstrate that in otherwise healthy individuals, hormonal shifts linked to fat storage typically occur after the onset of caloric surplus rather than initiating fat gain on their own.
Insulin Response: In controlled overfeeding trials, insulin levels increase in proportion to carbohydrate intake and overall energy surplus. These rises in fasting and postprandial insulin occur alongside increases in fat mass, suggesting that insulin’s storage-promoting effects are engaged as a reaction to the surplus (PMID: 7598063).
Leptin Adaptation: Overfeeding for as little as one week significantly increases circulating leptin levels, reflecting increased fat mass and energy availability. In one metabolic ward study, leptin rose by nearly 30% in response to surplus feeding without any baseline leptin resistance (PMID: 10799380).
Insulin Resistance Development: Chronic surplus feeding in healthy adults has been shown to impair insulin sensitivity within weeks. In a 3-week overfeeding study, insulin sensitivity dropped by roughly 17%, but only after sustained calorie surplus and concurrent weight gain (PMID: 10893333).
Cortisol and Other Stress Hormones: While cortisol can influence appetite and fat distribution, tightly controlled feeding studies do not show increases in cortisol driving fat gain without surplus. Elevated cortisol in free-living settings often reflects stress, poor sleep, or illness, but in the absence of surplus, significant fat gain is rare.
These findings align with the view that hormones act as mediators of fat storage once excess calories are present, rather than as autonomous initiators of weight gain in healthy individuals.
V. Special Populations and Exceptions
While caloric surplus is the primary initiator of fat gain in healthy individuals, certain medical conditions and genetic disorders can cause weight gain independent of deliberate overconsumption. Understanding these exceptions is important for accurate interpretation of the evidence.
Endocrine Disorders: Hypothyroidism reduces basal metabolic rate, which can lead to weight gain at calorie intakes that previously maintained weight. This effect occurs even if intake is not increased (PMID: 11502791). Cushing’s syndrome, characterized by chronic cortisol excess, can promote visceral fat accumulation through altered lipid metabolism (PMID: 20829625).
Genetic Syndromes: Conditions such as Prader–Willi syndrome involve hypothalamic dysfunction leading to hyperphagia and decreased energy expenditure. Even when intake appears modest, metabolic inefficiency and persistent hunger drive fat gain (PMID: 35449162).
Medication-Induced Weight Gain: Certain antipsychotics, antidepressants, and antiepileptic drugs can alter appetite signaling or reduce metabolic rate, resulting in fat gain without substantial increases in reported calorie intake (PMID: 38950403).
Fluid Retention vs. Fat Gain: Some medical conditions cause weight increase through edema rather than fat storage. Differentiating between these is essential for accurate attribution of weight gain causes.
These exceptions illustrate that while calorie surplus is the primary trigger in most healthy people, clinical and genetic factors can bypass the standard energy balance pathway, leading to fat accumulation without an apparent surplus.
VI. Longitudinal Observations
Evidence from prospective cohort studies and long-term follow-up trials supports the role of calorie surplus as the initiating factor for weight gain in free-living populations when medical conditions are absent.
Prospective Cohorts: In the Nurses’ Health Study, increases in daily energy intake over a 4-year interval were directly correlated with increases in body weight, even after adjusting for physical activity and baseline BMI (PMID: 21696306). Similar results were found in the Health Professionals Follow-Up Study, where participants with the largest calorie increases had the highest rates of fat mass gain over time (PMID: 21696306).
Free-Living Interventions: In a 12-month randomized trial examining dietary composition and weight change, participants assigned to diets of differing macronutrient ratios lost or gained weight in proportion to total calorie change, regardless of macronutrient distribution (PMID: 19246357).
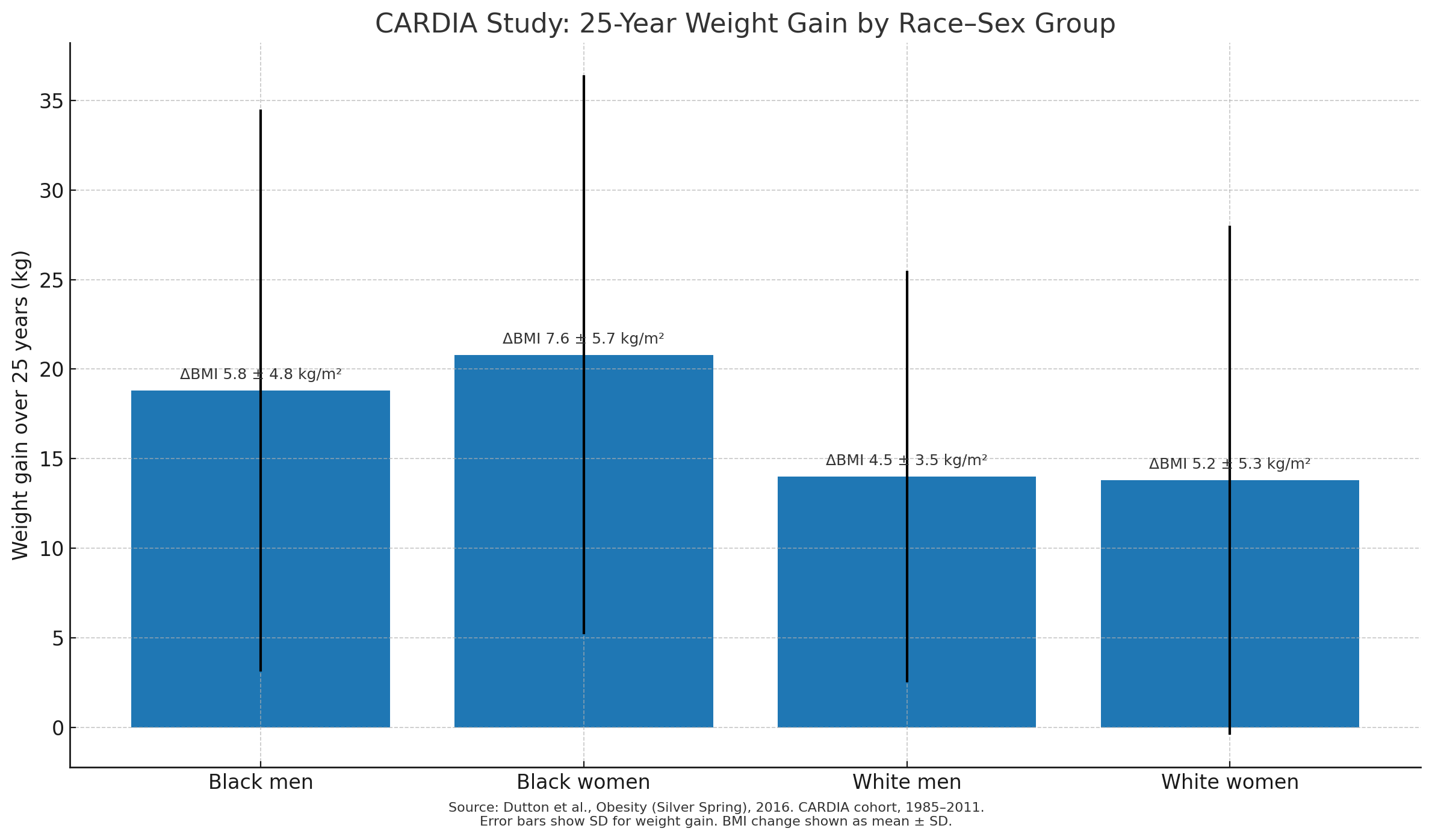
Energy Intake Tracking: Data from the CARDIA study, which followed participants for over two decades, revealed that cumulative increases in energy intake predicted gradual, sustained fat gain. The pattern held after accounting for activity levels, suggesting that sustained minor surpluses add up over time (PMID: 27569121).
Population-Level Trends: National dietary intake surveys in the United States have shown that the rise in average daily calorie consumption since the late 1970s parallels the increase in obesity prevalence, even after adjusting for demographic changes and activity levels (PMID: 18173389).
These long-term observational findings align with the metabolic ward and overfeeding data, indicating that in free-living conditions, sustained caloric surplus precedes and predicts weight gain across diverse populations.
VII. Synthesis of Findings
When the evidence from metabolic ward trials, controlled overfeeding studies, and long-term observational research is combined, a consistent pattern emerges: in healthy individuals without specific medical conditions, sustained caloric surplus is the initiating factor for measurable fat gain. Hormonal changes observed in these studies, such as increased insulin and leptin levels or decreased insulin sensitivity, appear after the surplus has been introduced, indicating they are downstream responses rather than primary triggers.
The table below summarizes key human trials that directly address the hypothesis:
| Study | Population | Design | Outcome | PMID |
|---|---|---|---|---|
| Bray et al., 2012 (JAMA) | Healthy adults (n=25) | 8-week overfeeding (+~1000 kcal/d) at low, normal, or high protein | All groups gained fat mass regardless of protein; lean mass ↑ with higher protein | 22215165 |
| Bray et al., 2015 (AJCN) | Men & women (n=25) | Metabolic-chamber overfeeding (~40% surplus) with 5%, 15%, 25% protein | EE rose with overfeeding; protein increased EE and lean mass more than fat | 25733634 |
| Acheson et al., 1988 | Healthy men | Carbohydrate overfeeding for 7 days | Rapid fat gain; marked ↑ in TG; evidence of de novo lipogenesis | 3165600 |
| Lammert et al., 2000 | Healthy young men | 21-day overfeeding (+~1200 kcal/d) with carb-rich vs fat-rich diets | Similar fat storage; leptin tracked fat gain; measurable de novo lipogenesis on high-carb | 11029975 |
| Tam et al., 2010 | Lean & overweight adults (n=36) | 28-day overfeeding (+1250 kcal/d; 45% fat) | Insulin sensitivity ↓ ~11% by clamp; CRP ↑; adipose macrophages unchanged | 20547978 |
| Swinburn, Sacks & Ravussin, 2009 | U.S. population data | Energy-supply vs weight trend analysis | Increased food energy supply is sufficient to explain U.S. weight gain | 19828708 |
Taken together, these studies reinforce that the onset of fat gain in otherwise healthy individuals requires an energy surplus. Hormonal changes, while important in regulating energy metabolism, follow the surplus and help manage its distribution, rather than serving as the initial cause of weight gain.
VIII. Practical Implications
The evidence from controlled feeding trials and long-term observational studies carries clear implications for both individual weight management and clinical practice.
For Individuals: Monitoring and managing total calorie intake remains the most reliable method for preventing fat gain in the absence of medical conditions that alter energy balance. While food quality, macronutrient composition, and meal timing can influence satiety, energy expenditure, and metabolic health, they cannot bypass the fundamental requirement of a caloric surplus to initiate fat gain. Individuals aiming to maintain or reduce fat mass benefit from tracking intake—whether through direct calorie counting, portion control, or structured meal planning—to avoid sustained surpluses.
For Clinicians: Counseling should emphasize energy balance while acknowledging that psychological, behavioral, and environmental factors influence calorie consumption. Teaching patients to recognize high-calorie-density foods, understand portion sizes, and identify habitual overeating patterns is essential. Clinicians should also be prepared to discuss exceptions, such as endocrine disorders or medication-induced changes, which may require different interventions.
Role of Macronutrient Strategies: Low-carbohydrate, low-fat, high-protein, and other dietary approaches can be tools for reducing calorie intake by increasing satiety or lowering energy density, but their effectiveness still depends on achieving and sustaining a calorie deficit when fat loss is desired.
Public Health Messaging: Nutrition guidelines and weight management programs should avoid framing hormones as the sole cause of weight gain without acknowledging the role of total energy intake. While hormones mediate storage, public messages should make it clear that over time, calorie surplus is the consistent initiator in healthy populations.
In practice, combining awareness of calorie balance with attention to nutrient quality offers the best protection against unintended fat gain and supports metabolic health across the lifespan.
IX. Limitations of Current Evidence
While the human trial data strongly support caloric surplus as the primary initiator of fat gain in healthy individuals, several limitations should be recognized when interpreting the findings.
Short Duration of Many Trials: Most metabolic ward and tightly controlled overfeeding studies last from a few days to several weeks. Although these provide precise measurements, they may not fully capture long-term adaptations such as metabolic downregulation, changes in non-exercise activity, or shifts in appetite regulation that occur over months or years.
Underreporting in Free-Living Studies: Large observational cohorts rely on self-reported dietary intake, which is prone to significant underestimation—particularly among individuals with overweight or obesity. This reporting bias can obscure the true magnitude of calorie surpluses or deficits in the data (PMID: 15531663).
Population Diversity: Many tightly controlled studies use small, relatively homogenous samples—often young, healthy adults—limiting generalizability to older adults, adolescents, or individuals from diverse ethnic and metabolic backgrounds.
Unmeasured Variables in Observational Data: While cohort studies adjust for known confounders, factors such as sleep quality, stress, environmental temperature, or untracked physical activity can influence energy balance and fat storage.
Rare Exceptions: Certain medical or genetic conditions can cause weight gain without overconsumption, as discussed earlier. These conditions are uncommon in the general population but represent important exceptions to the surplus-driven model.
Recognizing these limitations ensures that conclusions are drawn appropriately and that the role of calorie surplus is understood within the context of both controlled environments and the complexity of real-world living conditions.
X. Conclusion
The collective evidence from metabolic ward trials, controlled overfeeding experiments, and long-term observational studies strongly supports the hypothesis that in healthy individuals without clinical conditions affecting metabolism, the consumption of excess calories is the initiating step in fat storage and weight gain. Hormonal shifts such as increased insulin, elevated leptin, or reduced insulin sensitivity occur as downstream consequences of surplus intake, not as independent primary causes.
This distinction matters. It reframes the calorie–hormone debate by positioning hormones as responsive regulators within an energy balance framework, rather than autonomous drivers of fat gain in the absence of surplus. It also underscores the value of calorie awareness for both prevention and treatment of obesity, while leaving room for individualized dietary strategies to improve adherence and metabolic health.
From a practical perspective, the findings reinforce that:
Fat gain requires an energy surplus under normal physiological conditions.
Macronutrient composition and hormonal responses influence how the surplus is processed, but do not negate the need for surplus to initiate fat gain.
Exceptions exist in specific medical, genetic, or pharmacological scenarios, but these are relatively rare in the general population.
Ultimately, the science points to a straightforward starting point for weight control: prevent or eliminate sustained calorie surpluses. Whether achieved through dietary adjustments, increased activity, or a combination of both, maintaining energy balance remains the most reliable defense against unwanted fat accumulation in otherwise healthy individuals.
Updated: August 13, 2025 10:19
Category: Science
Keywords: calorie surplus fat storage weight gain
References
Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307(1):47–55. PMID: 22215165. https://pubmed.ncbi.nlm.nih.gov/22215165
Bray GA, Redman LM, de Jonge L, et al. Effect of protein overfeeding on energy expenditure measured in a metabolic chamber. Am J Clin Nutr. 2015;101(3):496–505. PMID: 25733634. https://pubmed.ncbi.nlm.nih.gov/25733634
Horton TJ, Drougas H, Brachey A, et al. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62(1):19–29. PMID: 7598063. https://pubmed.ncbi.nlm.nih.gov/7598063
Lammert O, Grunnet N, Faber P, et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84(2):233–245. PMID: 11029975. https://pubmed.ncbi.nlm.nih.gov/11029975
Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. PMID: 7632212. https://pubmed.ncbi.nlm.nih.gov/7632212
Levine JA, Eberhardt NL, Jensen MD. Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. J Clin Endocrinol Metab. 1999;84(8):2751–2754. PMID: 10443673. https://pubmed.ncbi.nlm.nih.gov/10443673
Samocha-Bonet D, Campbell LV, Mori TA, et al. Overfeeding reduces insulin sensitivity and increases oxidative stress, without altering markers of mitochondrial content and function in humans. PLoS ONE. 2012;7(5):e36320. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0036320
Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. PMID: 21696306. https://pubmed.ncbi.nlm.nih.gov/21696306
Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48(10):373–379. PMID: 2082216. https://pubmed.ncbi.nlm.nih.gov/2082216
Bleich SN, Cutler DM, Murray CJL, Adams A. Why is the developed world obese? Annu Rev Public Health. 2008;29:273–295. PMID: 18173389. https://pubmed.ncbi.nlm.nih.gov/18173389
Comments
You must log in to post a comment.